You are here
Check4 has moved from the development stage to the commercialization stage. Lear more in the company's news release.
https://www.prnewswire.com/news-releases/identifysensors-biologics-moves-new-pathogen-testing-platform-to-commercialization-stage-301291239.html
I received an email about the seminar this week and I erased the email by accident. Can you please send me the link to the seminar that is happening this week?
I received an email about the seminar this week and I erased the email by accident. Can you please send me the link to the seminar that is happening this week?
We post all of our videos to YouTube at https://www.youtube.com/channel/UCoF95noAZpYl3SysSKe7d7Q/videos
We post all of our videos to YouTube at https://www.youtube.com/channel/UCoF95noAZpYl3SysSKe7d7Q/videos
Based on high demand from institutional investors and family offices, IdentifySensors Biologics now offers a Regulation D opportunity in this same investment for larger investors. For immediate access to a senior investment advisor please call 737-757-8067 or follow this link: https://www.manhattanstreetcapital.com/contact/offering/nojs/25045
Based on high demand from institutional investors and family offices, IdentifySensors Biologics now offers a Regulation D opportunity in this same investment for larger investors. For immediate access to a senior investment advisor please call 737-757-8067 or follow this link: https://www.manhattanstreetcapital.com/contact/offering/nojs/25045
Purdue University last week (February 1) invested a substantial amount of money into this technology. The university is very close to sending the prototype to the FDA for emergency use authorization. Once this happens, the lab will redirect development for additional test cartridges that use the same reusable reader. These cartridges will test for pathogens such as Influenza A, B and C. More to follow.
Purdue University last week (February 1) invested a substantial amount of money into this technology. The university is very close to sending the prototype to the FDA for emergency use authorization. Once this happens, the lab will redirect development for additional test cartridges that use the same reusable reader. These cartridges will test for pathogens such as Influenza A, B and C. More to follow.
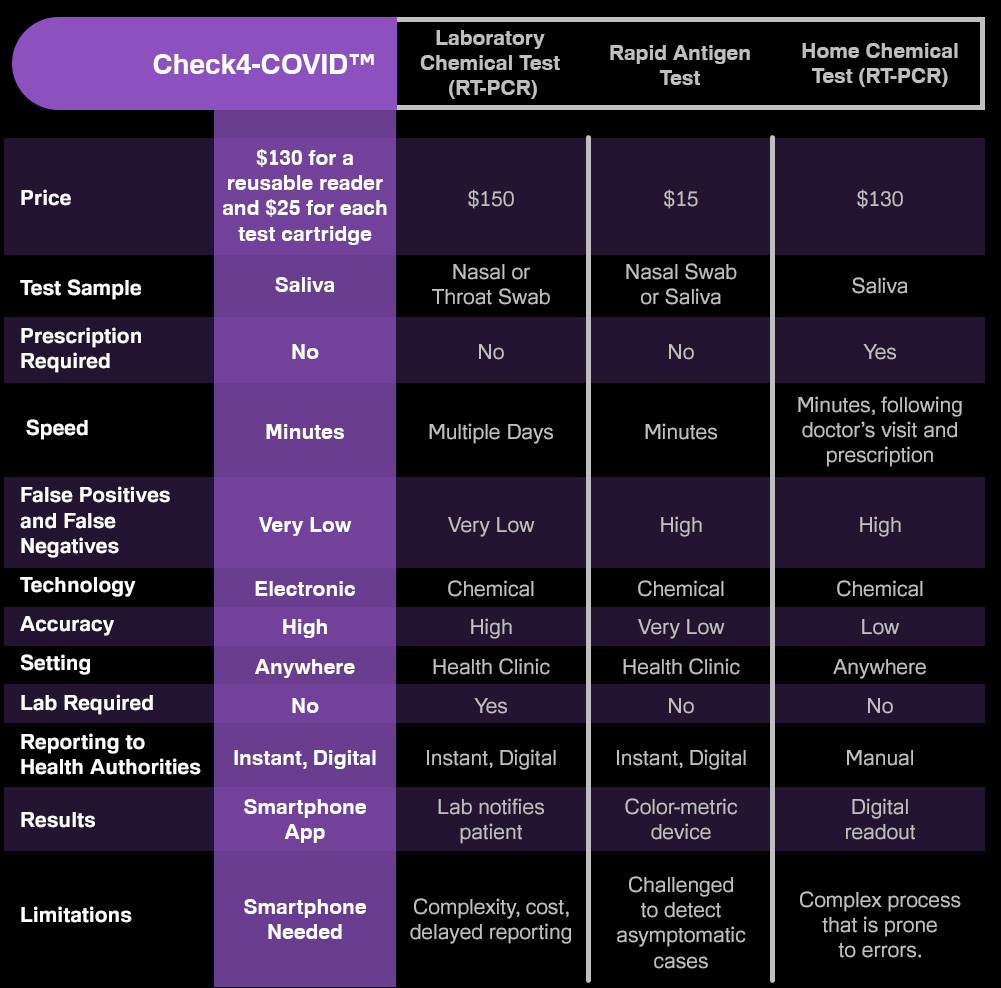
We're receiving questions from investors about the new COVID variants and if this test can detect them. The test is vary far along in the development stage and recent trials indicate that this test intends to detect variant strains well. Because the test is based on an electronic signal and not a chemical reaction, the variants are easily identified.
We're receiving questions from investors about the new COVID variants and if this test can detect them. The test is vary far along in the development stage and recent trials indicate that this test intends to detect variant strains well. Because the test is based on an electronic signal and not a chemical reaction, the variants are easily identified.
We've been asked by potential investors if a new COVID test is late in the pandemic, considering that vaccines now are rolling out. Experts worldwide agree that vaccines alone will not end the pandemic. Testing will continue to play a critical role in slowing the spread and beyond. Furthermore, there is an overwhelming need for an accurate rapid test free from barriers such as prescriptions, mailing in samples and high costs. COVID-19 and its emerging variants will likely be a part of life forever, similar to the flu. Many virologists agree that COVID will eventually become more manageable, similar to the flu, but like the flu, testing will remain essential, especially for more vulnerable people. IdentifySensors is creating a platform intended to test for many pathogens beyond COVID and the variants, including the flu, MRSA, HIV and other common infections. This is all-new technology intended to disrupt the entire diagnostics industry, much the way pregnancy tests did in the 1960s.
We've been asked by potential investors if a new COVID test is late in the pandemic, considering that vaccines now are rolling out. Experts worldwide agree that vaccines alone will not end the pandemic. Testing will continue to play a critical role in slowing the spread and beyond. Furthermore, there is an overwhelming need for an accurate rapid test free from barriers such as prescriptions, mailing in samples and high costs. COVID-19 and its emerging variants will likely be a part of life forever, similar to the flu. Many virologists agree that COVID will eventually become more manageable, similar to the flu, but like the flu, testing will remain essential, especially for more vulnerable people. IdentifySensors is creating a platform intended to test for many pathogens beyond COVID and the variants, including the flu, MRSA, HIV and other common infections. This is all-new technology intended to disrupt the entire diagnostics industry, much the way pregnancy tests did in the 1960s.
We were recently asked if buyers can purchase IdentifySensors Biologics stock through a broker? Unfortunately, you cannot. Buyers can only purchase through this site. However, buyers can use money in their brokerage account, but a broker cannot make the purchase on someone else's behalf.
We were recently asked if buyers can purchase IdentifySensors Biologics stock through a broker? Unfortunately, you cannot. Buyers can only purchase through this site. However, buyers can use money in their brokerage account, but a broker cannot make the purchase on someone else's behalf.
We were asked about liquidity: The company intends to list the Regulation A+ shares on one of the Regulation A+ aftermarkets when the offering has completed. This is called Alternative Trading System or ATS. We are in the process of evaluating aftermarket options that will work best for our investors.
These aftermarkets allow Reg A+ investors to buy and sell their securities. Because these markets are relatively new, we do not yet know how much liquidity they will provide. In the future we may elect to list the company on a major exchange like the NASDAQ. There can be no assurance that such a listing will occur.
We were asked about liquidity: The company intends to list the Regulation A+ shares on one of the Regulation A+ aftermarkets when the offering has completed. This is called Alternative Trading System or ATS. We are in the process of evaluating aftermarket options that will work best for our investors.
These aftermarkets allow Reg A+ investors to buy and sell their securities. Because these markets are relatively new, we do not yet know how much liquidity they will provide. In the future we may elect to list the company on a major exchange like the NASDAQ. There can be no assurance that such a listing will occur.
We were asked if these shares will pay dividends. At this time, we expect the company will invest any profits back into its business. However, if the operation of the company produces sufficient profits that the Board of Directors determines the company should make distributions of profits to shareholders, the company will make dividend distributions to it’s Reg A+ shareholders.
We were asked if these shares will pay dividends. At this time, we expect the company will invest any profits back into its business. However, if the operation of the company produces sufficient profits that the Board of Directors determines the company should make distributions of profits to shareholders, the company will make dividend distributions to it’s Reg A+ shareholders.
Offering Circular
Please read the Offering Circular here: Get Offering Circular
AN OFFERING STATEMENT REGARDING THIS OFFERING HAS BEEN FILED WITH THE SEC. THE SEC HAS QUALIFIED THAT OFFERING STATEMENT, WHICH ONLY MEANS THAT THE COMPANY MAY MAKE SALES OF THE SECURITIES DESCRIBED BY THE OFFERING STATEMENT. IT DOES NOT MEAN THAT THE SEC HAS APPROVED, PASSED UPON THE MERITS OR PASSED UPON THE ACCURACY OR COMPLETENESS OF THE INFORMATION IN THE OFFERING STATEMENT. YOU MAY OBTAIN A COPY OF THE OFFERING CIRCULAR THAT IS PART OF THAT OFFERING STATEMENT FROM:
https://www.
YOU SHOULD READ THE OFFERING CIRCULAR BEFORE MAKING ANY INVESTMENT.






















Comments
Post comment